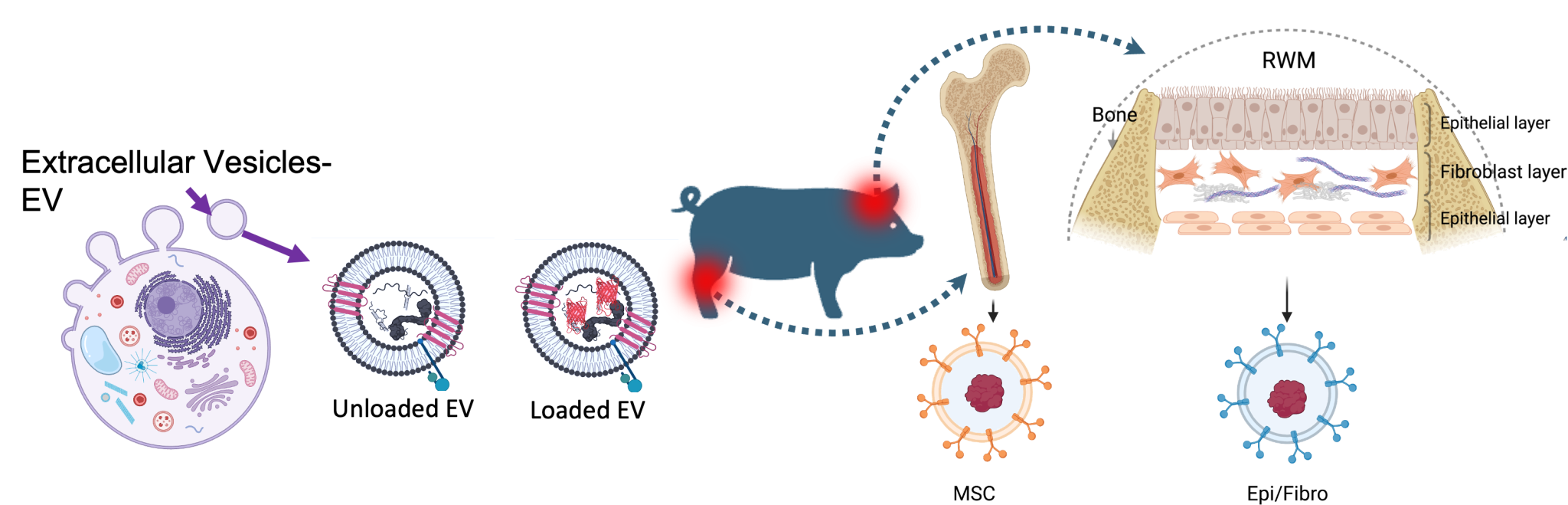
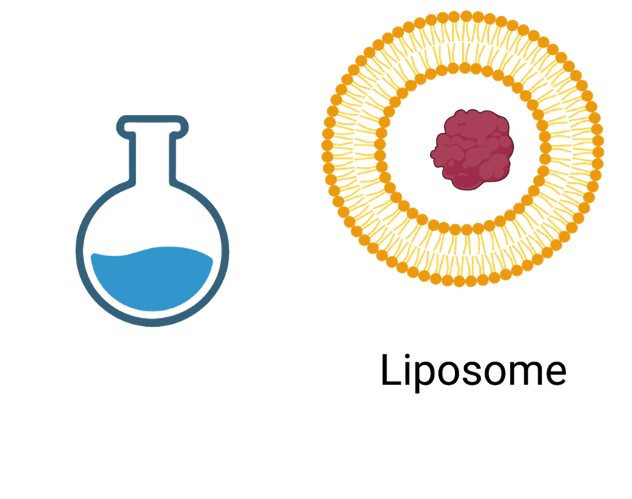Developing extracellular vesicles as efficacious and safe carriers to deliver therapy-related substances to the inner ear.
- Extracellular vesicles, exosomes
-
Membrane-bound extracellular nanovesicles (exosomes) that are produced in the endosomal compartment of most eukaryotic cells possess membrane features and intra-vesicular molecular components that could provide superior characteristics for cargo delivery compared to synthetic nanoparticles. Exosomes contain two lipid layer membranes covered with diverse ligands contributing to interactions with target cells. During transit through the extracellular environment, exosomes protect their cargo from enzymatic degradation, thus improving their half-life inside the inner ear. They naturally transport cargo between cells, elicit a minimal immune response, and are stable in blood circulation. Exosomes have a membrane structure more like the original cell in terms of lipid composition, fluidity, membrane proteins, and targeting potential. Thus, exosomes have a higher affinity for uptake by cells of the same type as their parent cell.
Exosomes are currently being used in clinical trials for metastatic melanoma, advanced non-small cell lung cancer, colorectal cancer, and malignant glioma. The success of exosome-based therapies in Phase I clinical trials demonstrates their safety and feasibility for clinical use.
We evaluate and compare the uptake (by porcine RWM cells in culture) and passage (across porcine RWM explants) efficiency of the native exosomes (exosomes secreted by the porcine RWM cells) and non-native exosomes (exosomes released by the porcine bone marrow mesenchymal stem cells--MSC).
Loading different therapeutic substances inside the vesicles:
- Proteins such as BDNF
- Viral vectors (AAVs)
- Small molecules such as dexamethasone
- mRNAs
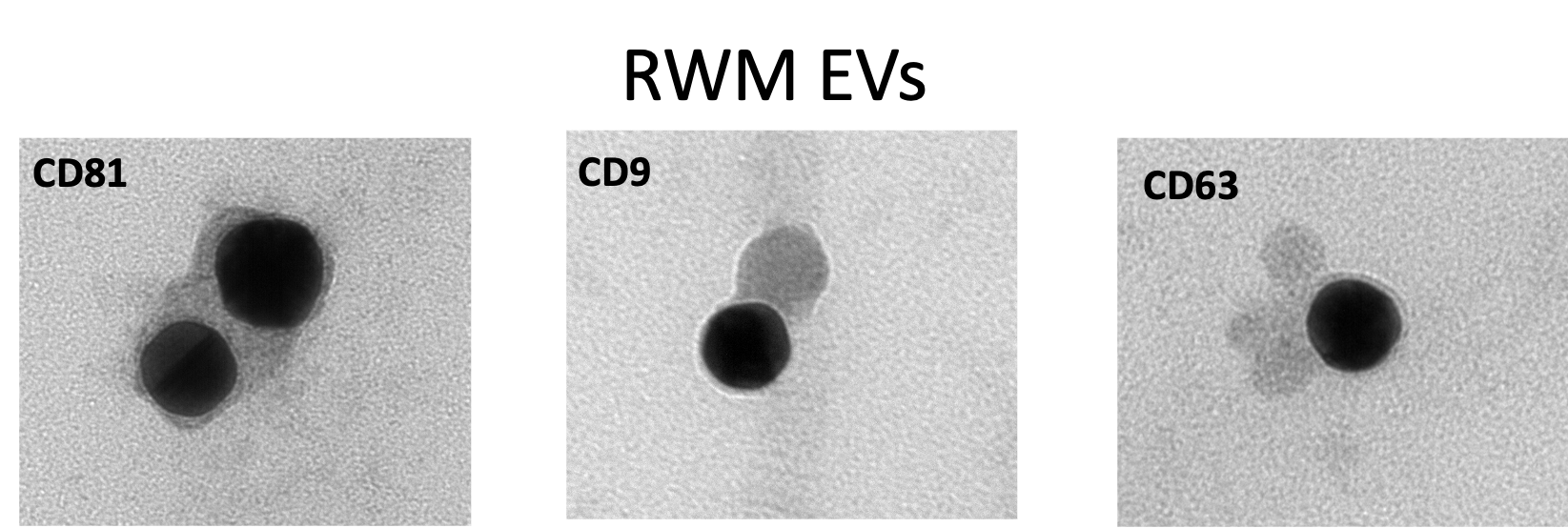
Transmission electron microscopy-TEM micrographs of exosomes released by RWM cells illustrating the exosome markers (CD81, CD9, and CD63 conjugated with gold nanoparticles):
- Engineered lipososmes
-
Liposomes are tiny spherical vesicles composed of lipid bilayers that encapsulate substances. Their structure is similar to cell membranes, made up of phospholipids, which form a double-layered membrane. These properties make liposomes ideal for delivering drugs, nutrients, or other therapeutic agents directly to target cells, as they have been used for mRNA Covid-19 vaccine delivery. However, to optimize their passage, they need to be engineered for the specific membrane (here RWM) and target cells (here inner ear cells).
Engineering liposomes via:
- Matching their lipofluidity
- Adding peptide tags to improve passage and targetability